How do I build a process template?
Use Process Builder to provide sequential steps to your staff to complete compliant forms
A major requirement of FDA and CFIA regulations is to determine the "Readiness for commercial manufacturing" and the "conformation to application" of the manufacturers. In simple terms, this basically translates to (1) are you set up to be compliant and (2) are you following your compliance set up.
Weever Process is designed to help you efficiently manage your food safety CGMP compliance requirements by ensuring your operating procedures are followed to the letter. Managers simply build Process Templates that are launched by staff every time a new batch is started. Process Templates consist:
- The forms that need to be completed
- Steps & Tasks that define the process
- Staff roles required to launch given tasks
- Specific fields required to complete a give task
To create a new Process Template, follow these steps:
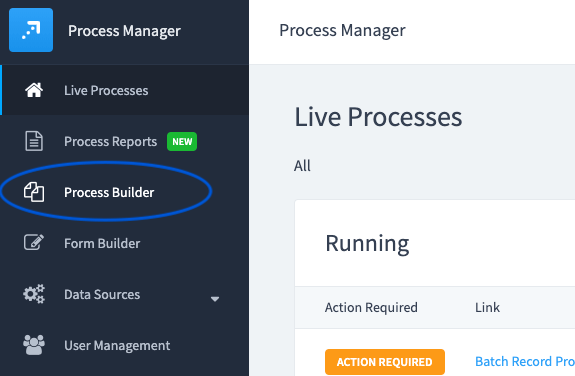
Step 1:
Select "Process Builder" in the main menu.
Step 2:
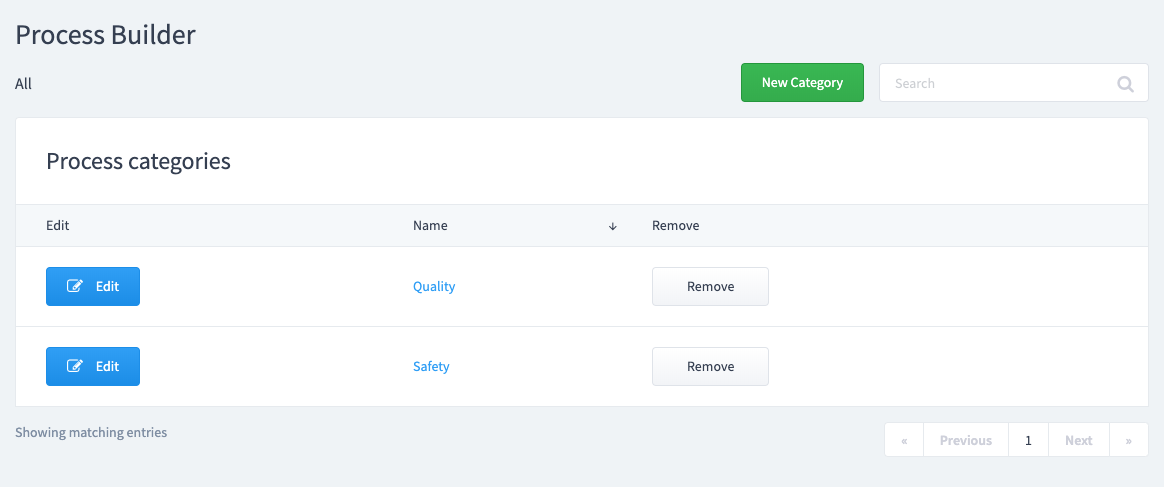
Choose a category for your process template.
Process categories allow you to organize your processes. Create a "New Category" or select an existing one.
Step 3:
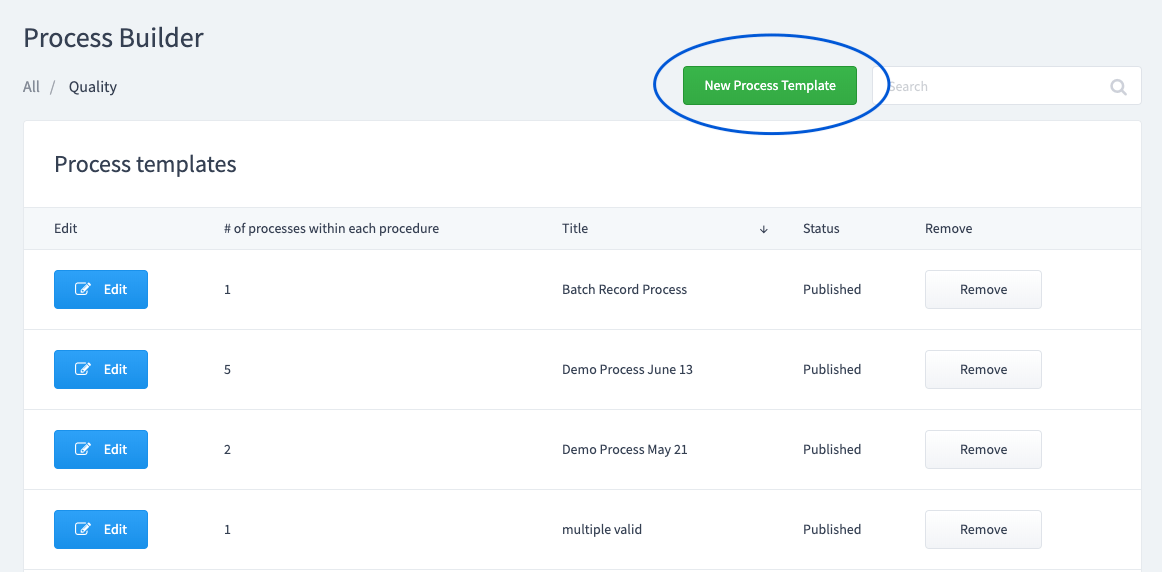
Create or select a Summary Form.
This is the information you want to collect when the process is initiated. This information will be associated automatically with any form that is added as a task to this process.
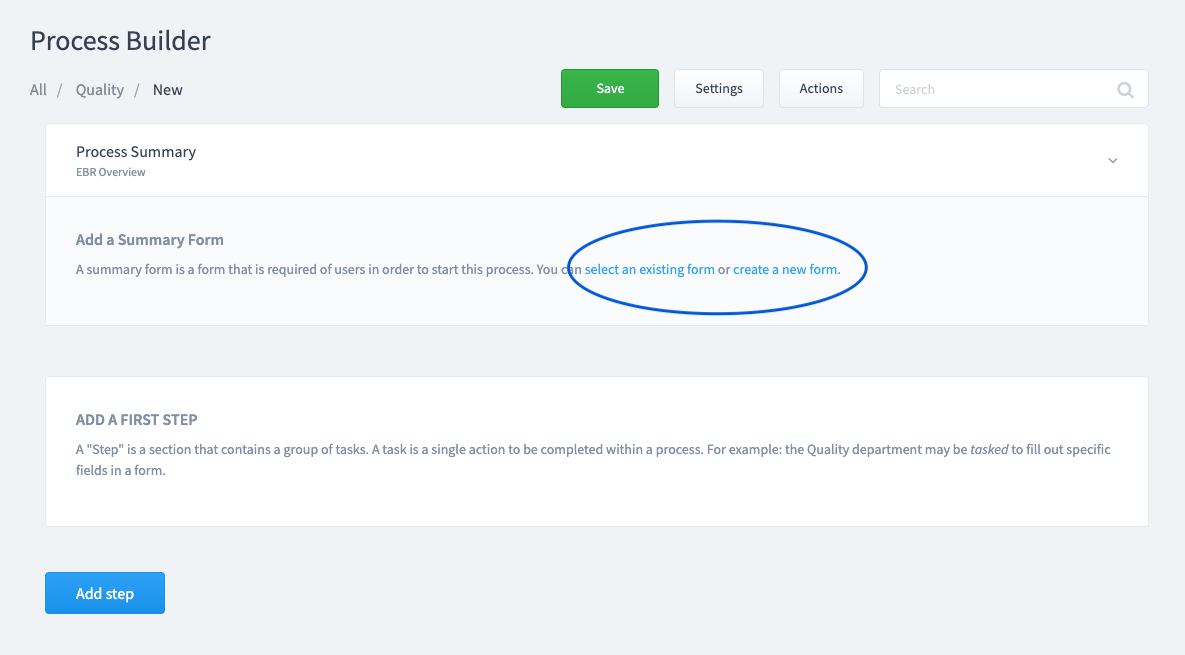
Step 4:
Add a Step
A "Step" is a section that contains a group of tasks.
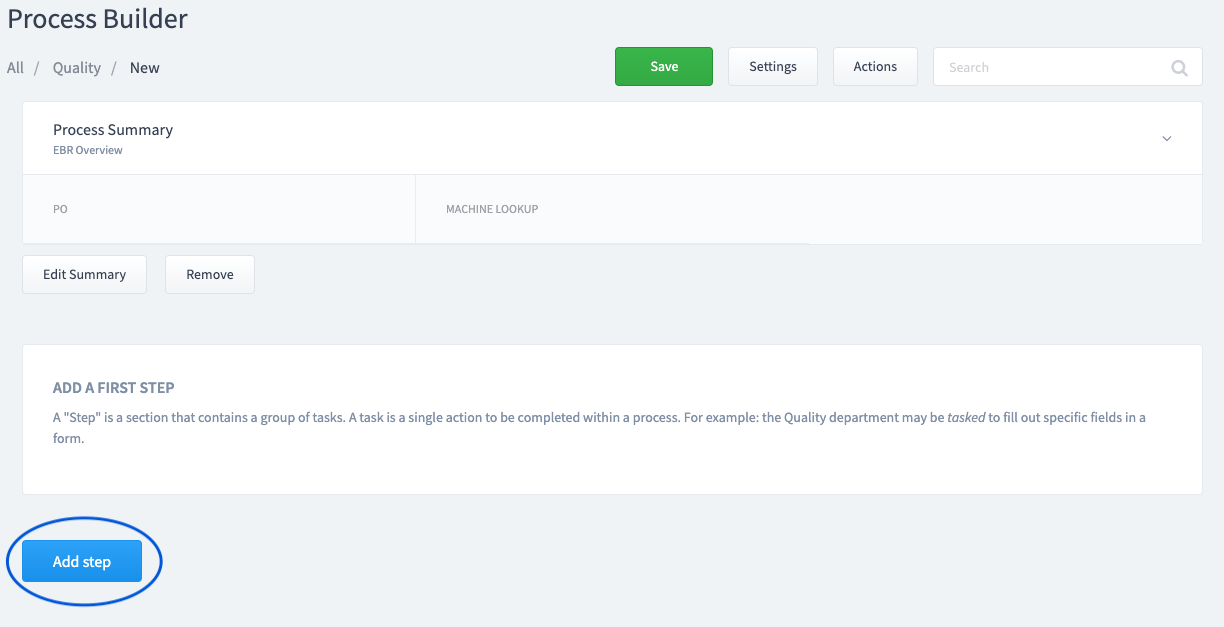
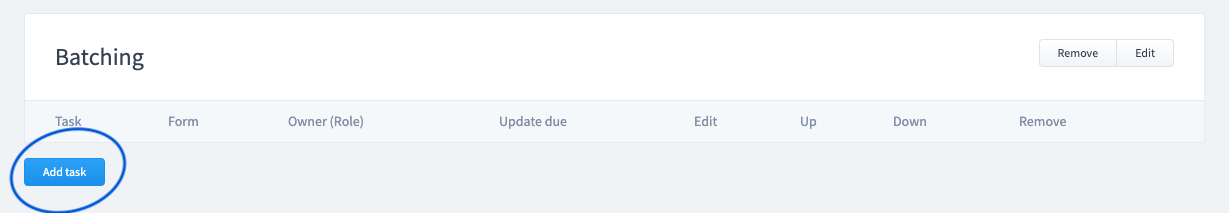
Step 5:
Add Tasks
A task is a single action to be completed within a process. For example: the Quality department may be tasked to fill out specific fields in a form.
- Tasks can be required multiple times over a time period (repeated).
- Tasks can be associated to roles so only certain staff can initiate the task.
- Tasks can be associated to specific fields within a form.